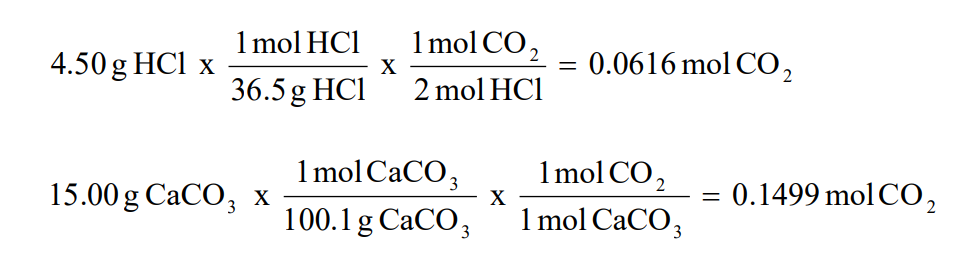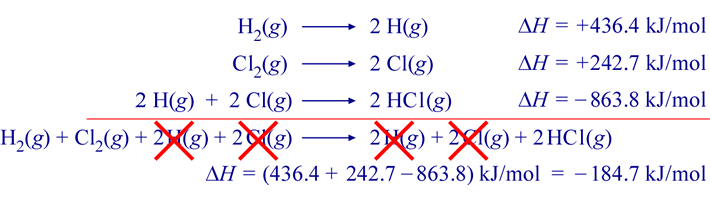32 The dissociation of CO2 can be expressed as 2CO2 converted into 2CO +O2. If 2 mol of CO2 is taken initially and 40
2 mol of Hg(g) is combusted in a fixed volume bomb calorimeter with excess of O2 at 298 K - Sarthaks eConnect | Largest Online Education Community
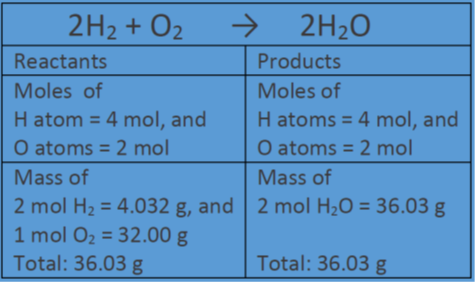
2 moles of an ideal gas at temp 27 degree c is heated isoermall from volume v to 4v .if R=2 cal/mol then the heat input in the process is approximately

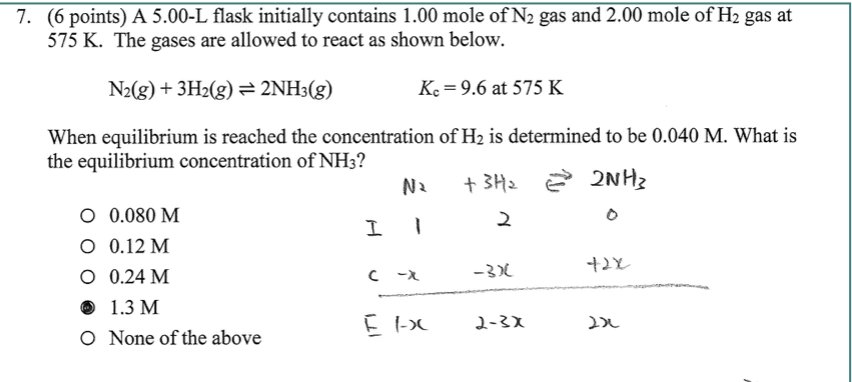
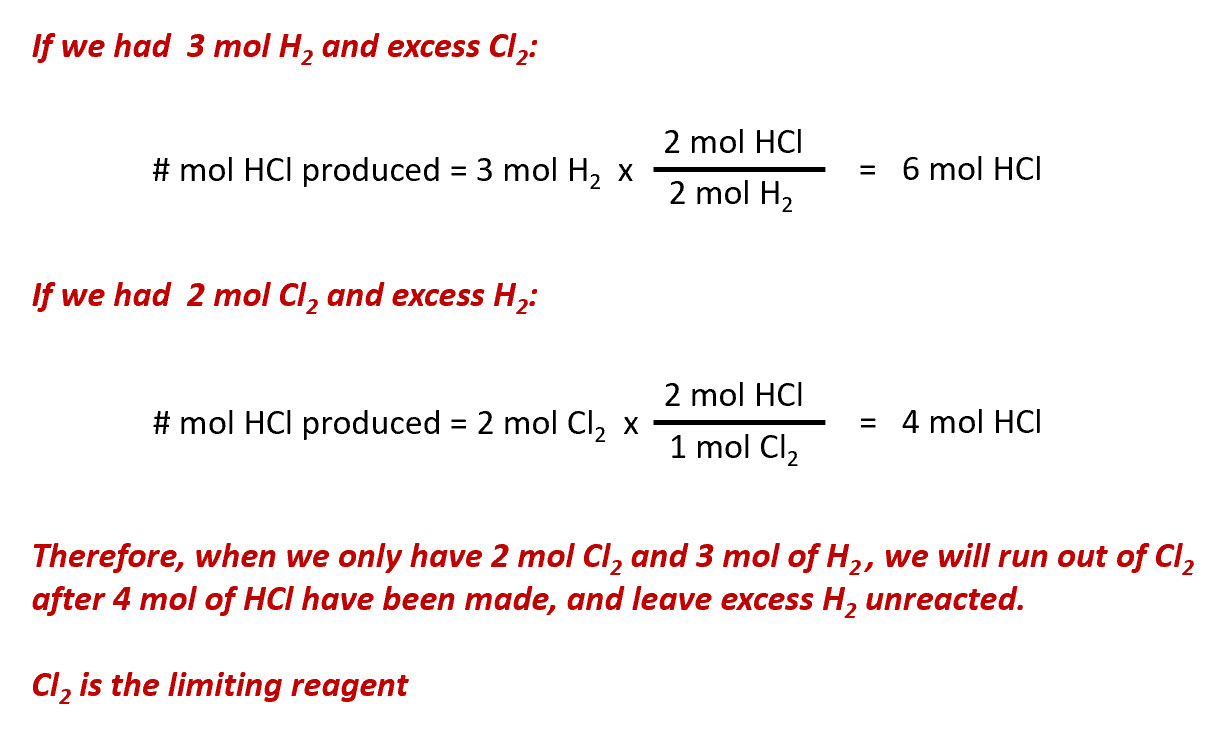
1 mole of N2 and 2 moles of H2 are allowed to react in a 1 dm^3 vessel. At equilibrium 0.8 mole of NH3 is formed. The concentration of H2 in the vessel is

Question Video: Identifying a Precipitating Agent for the Gravimetric Analysis of Chloride Ions | Nagwa

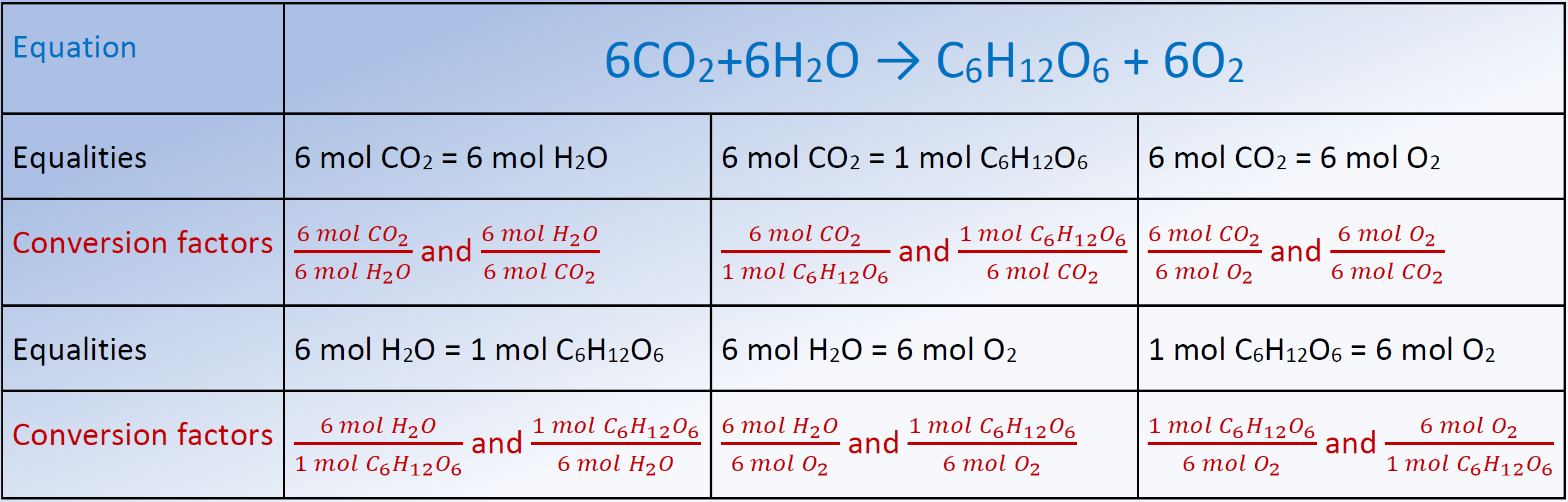
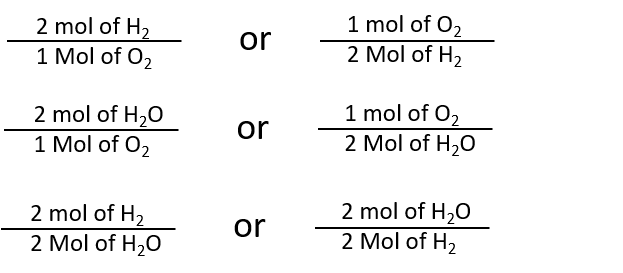
How to Find Moles of Product from Moles of Reactant using a Chemical Equation | Chemistry | Study.com