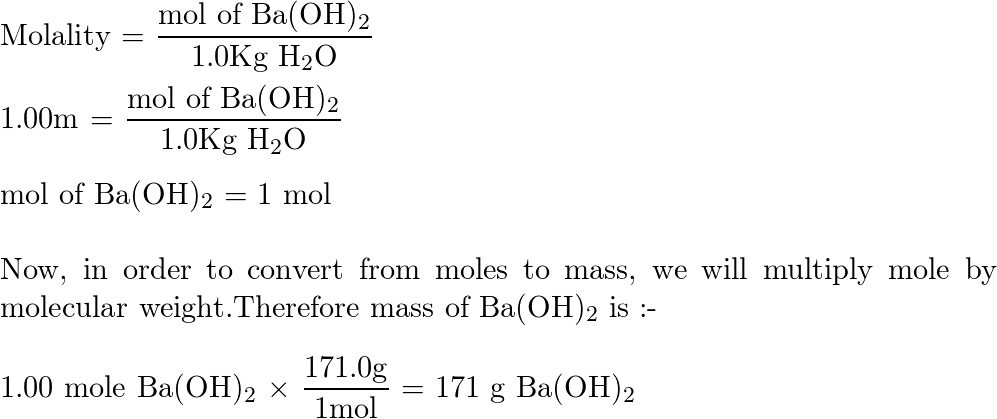BALANCE THIS GIVEN EQUATION BY THE HELP OF THE ALTERNATE WAY OF BALANCING NOT THE TRADITIONAL WAY BY SHOWING THE APPROPRIATE STEPS ELABORATELY. EQN : Ba(OH)2 + NH4Cl —> BaCl2 + NH3 + H2O

SOLVED: Consider the unbalanced equation for the neutralization of acetic acid: HC2H3O2(aq) + Ba(OH)2(aq)-H2O(l ) + Ba(C2H3O2)2(aq) Balance the equation and determine how many moles of Ba(OH)2 are required to completely neutralize

Question Video: Determining the Products of the Neutralization Reaction of Barium Hydroxide Ba(OH)₂ with Carbonic Acid H₂CO₃ | Nagwa

The solubility of Ba (OH)2 . 8H2O in water ar 288K is 5.6g per 100g of water. What is the molality of the hydroxide ions in saturated solution of Ba (OH)2 .