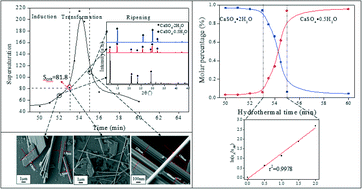
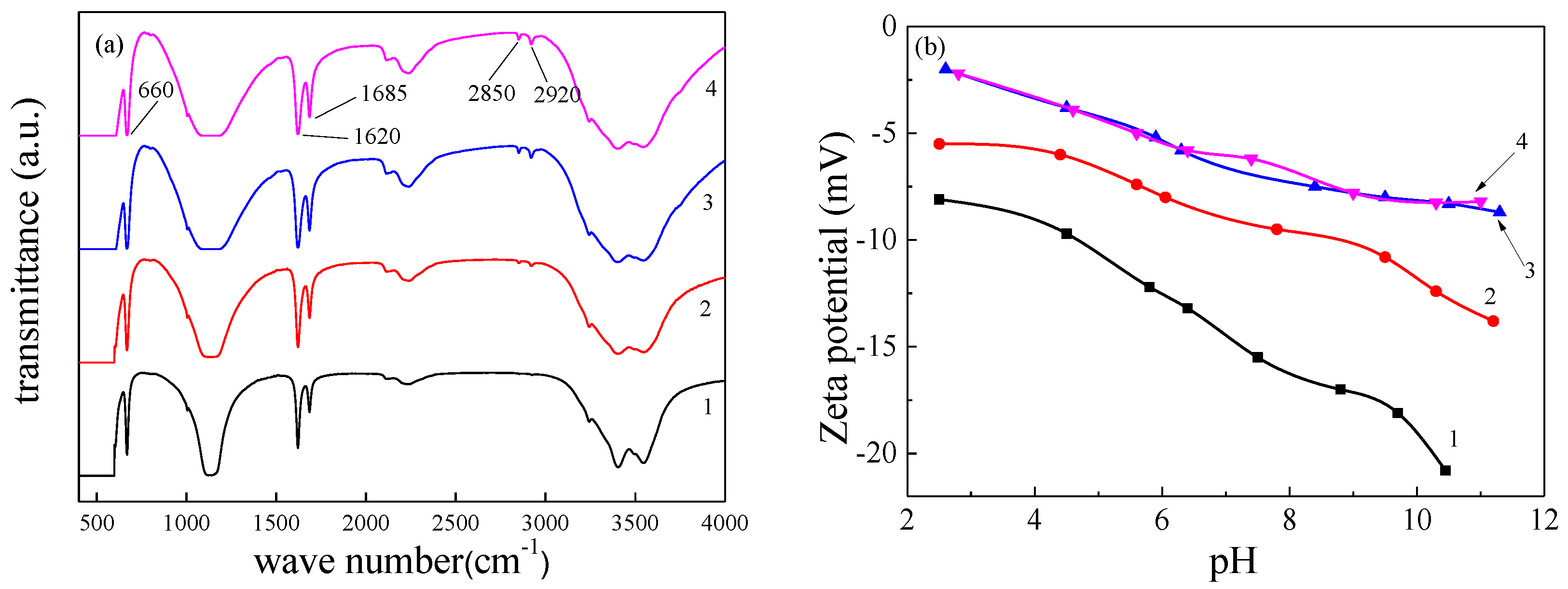
Crystals | Free Full-Text | Effect of Molecular Structure of Organic Acids on the Crystal Habit of α-CaSO4·0.5H2O from Phosphogypsum
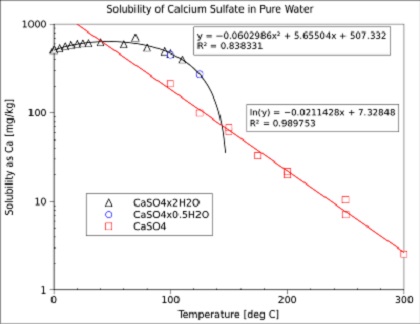
![PDF] Thermodynamic Modeling of Calcium Sulfate Hydrates in the CaSO4–H2O System from 273.15 to 473.15 K with Extension to 548.15 K | Semantic Scholar PDF] Thermodynamic Modeling of Calcium Sulfate Hydrates in the CaSO4–H2O System from 273.15 to 473.15 K with Extension to 548.15 K | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/7cebe8c890fa31a7af18365a4240a254e5398399/2-Table1-1.png)
PDF] Thermodynamic Modeling of Calcium Sulfate Hydrates in the CaSO4–H2O System from 273.15 to 473.15 K with Extension to 548.15 K | Semantic Scholar
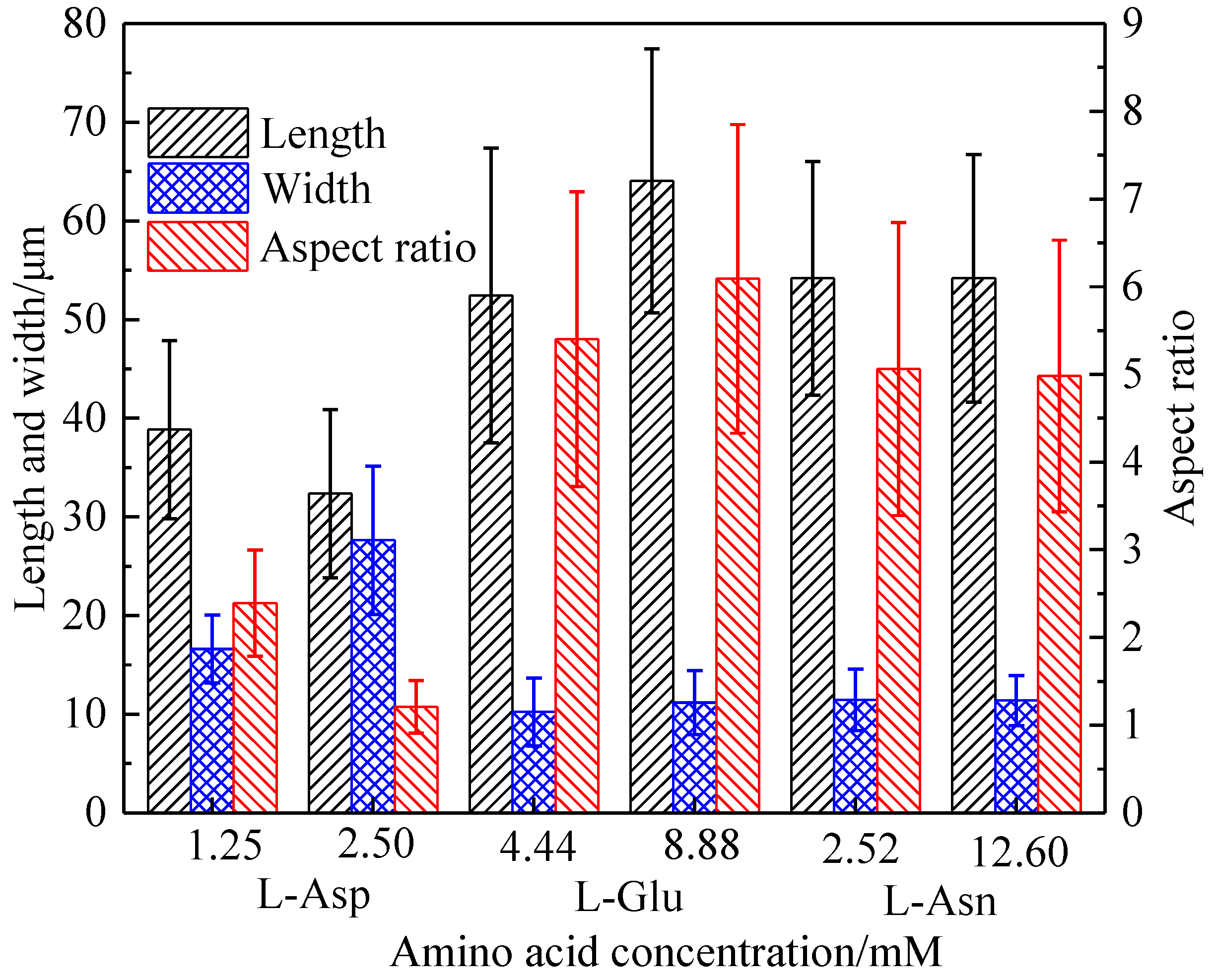
Crystals | Free Full-Text | Influence of Alkyl Trimethyl Ammonium Bromides on Hydrothermal Formation of α-CaSO4·0.5H2O Whiskers with High Aspect Ratios
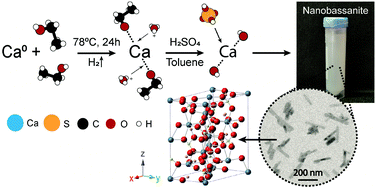
Synthesis of high surface area CaSO4·0.5H2O nanorods using calcium ethoxide as precursor - Chemical Communications (RSC Publishing)

Formation and Transformation of Five Different Phases in the CaSO4−H2O System: Crystal Structure of the Subhydrate β-CaSO4·0.5H2O and Soluble Anhydrite CaSO4 | Chemistry of Materials
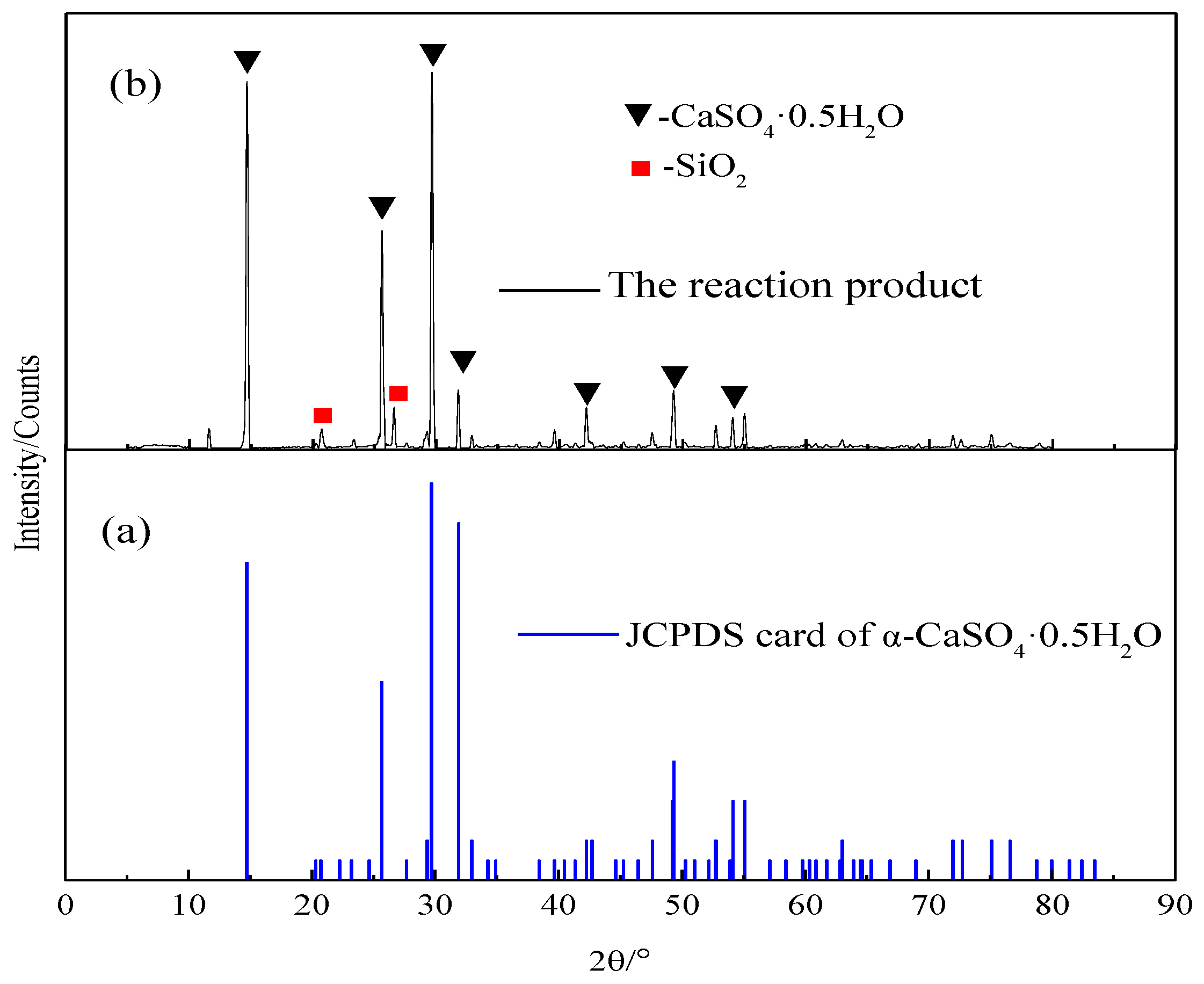
Crystals | Free Full-Text | Effect of Molecular Structure of Organic Acids on the Crystal Habit of α-CaSO4·0.5H2O from Phosphogypsum

Crystals | Free Full-Text | Influence of Alkyl Trimethyl Ammonium Bromides on Hydrothermal Formation of α-CaSO4·0.5H2O Whiskers with High Aspect Ratios

Direct synthesis of single-phase α-CaSO4·0.5H2O whiskers from waste nitrate solution - ScienceDirect

PDF) Bassanite (CaSO4·0.5H2O) dissolution and gypsum (CaSO4·2H2O) precipitation in the presence of cellulose ethers
Force field for calcium sulfate minerals to predict structural, hydration, and interfacial properties

Formation and Transformation of Five Different Phases in the CaSO4−H2O System: Crystal Structure of the Subhydrate β-CaSO4·0.5H2O and Soluble Anhydrite CaSO4 | Chemistry of Materials