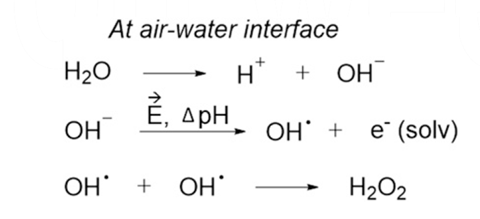
Direct production of H2O2 from H2 and O2 in a biphasic H2O/scCO2 system over a Pd/C catalyst: Optimization of reaction conditions - ScienceDirect
1) H2O2 + O3 → H2O +2O2 2)H2O2 +Ag2O →2Ag +H2O +O2 Determine whether H2O2 is oxidised or reduced in the above reaction? Explain.
Free Online Help: Given the following delta H values H2+1/2O2--->H2O delta H =-285.8 H2O2---->H2+O2 delta H = 187.6 Calculate delta H rxn for the following reaction H2O2--->H2O + 1/2O2
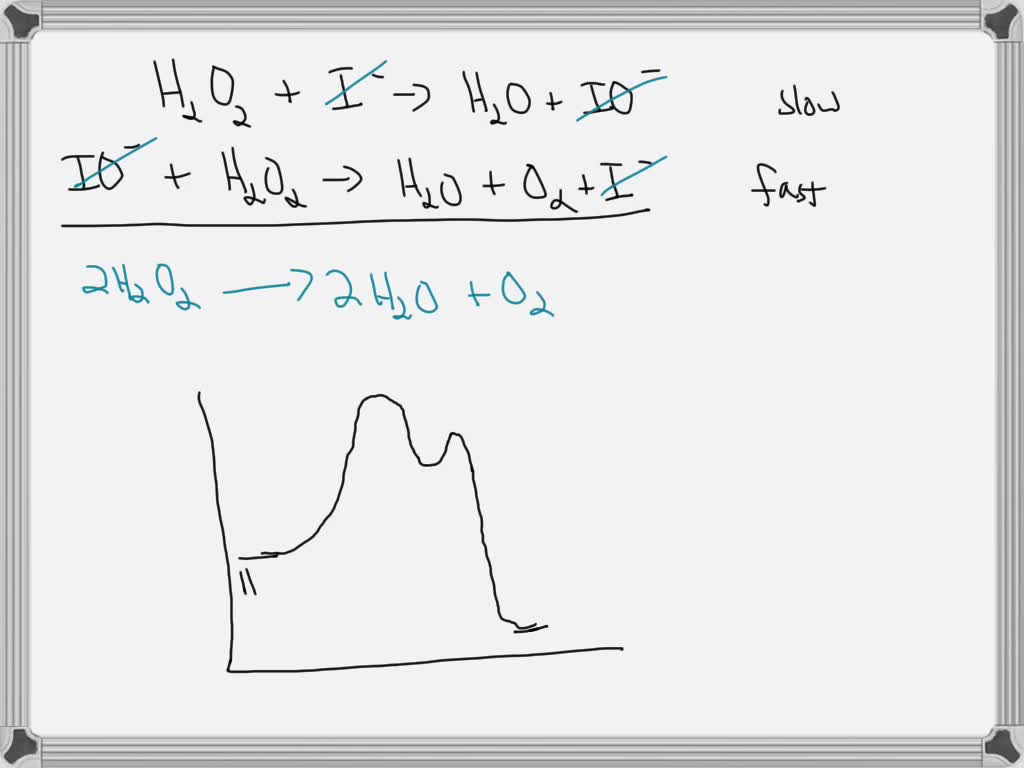
Why is the answer B? Can someone explain this to me and why other options are incorrect. I assumed that H2O2 will decompose rapidly to form H20 and O2 with MnO2 as

The decomposition of H2O2 has a strong thermodynamic driving force 2H2O2→ 2H2O + O2(g) Δ H = - 99kj/mole,Δ s = + 69JK^-1 mole^-1 Addition of solution of KI causes H2O2 to
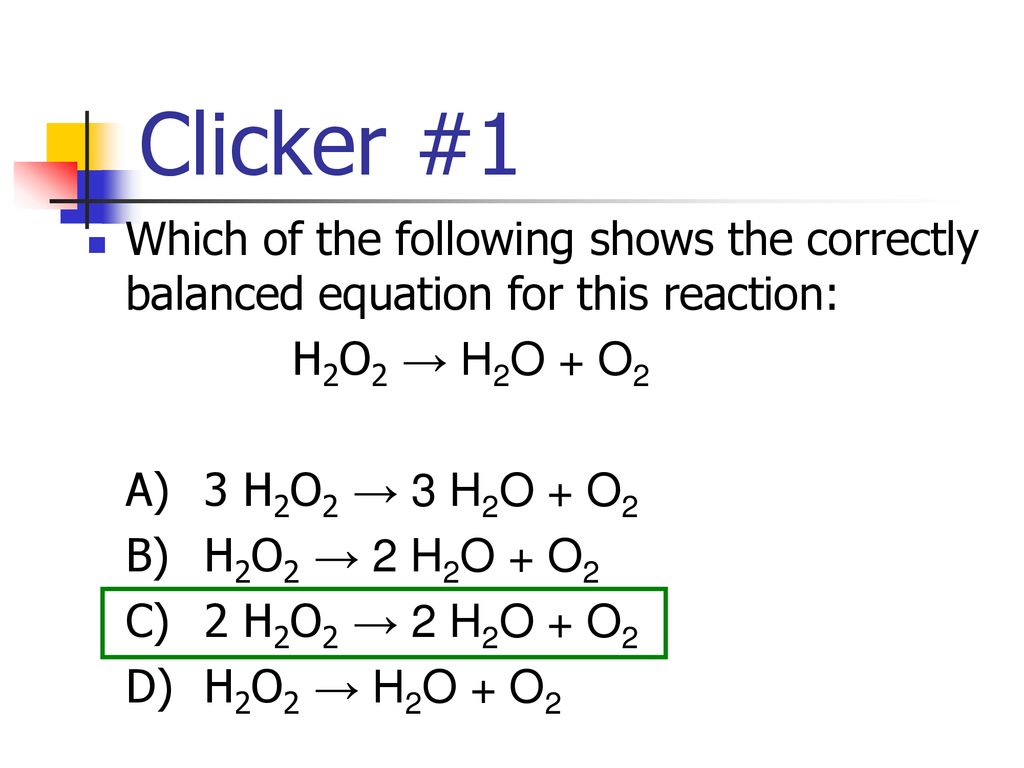
Clicker #1 Which of the following shows the correctly balanced equation for this reaction: H2O2 → H2O + O2 A) 3 H2O2 → 3 H2O + O2 B) H2O2 → 2 H2O + O2. - ppt download
![For the reaction ; 2H2O2(aq)→ 2H2O(l) + O2(g) , rate of decomposition for H2O2 = k[H2O2]^2 For the reaction ; 2H2O2(aq)→ 2H2O(l) + O2(g) , rate of decomposition for H2O2 = k[H2O2]^2](https://dwes9vv9u0550.cloudfront.net/images/2785739/3a8762db-6023-4a5c-9588-862513a5c19d.jpg)